ark5230
Advanced Member level 3

- Joined
- Jun 29, 2009
- Messages
- 862
- Helped
- 163
- Reputation
- 324
- Reaction score
- 140
- Trophy points
- 1,323
- Location
- India
- Activity points
- 6,187
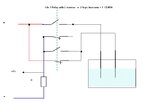
I am trying to measure resistance of NaCl solution using conductivity cell 1 cm x 1cm.
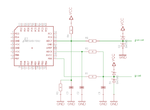
The cell is connected to +5V supply of Atmega16 via 47K.
The other end of cell is ground.
Measuring voltage across cell did not give meaningful results.
I tried changing 47K to 200K on higher side in steps, and lower side to 10K.
In all the cases there is very marginal change in readings.
Is the input impedance of the ADC responsible for this as the resistance of the cell with solution is expected to be in the range of 20K to 300K.
Any clue?
Is the electrolyte nature of solution the culprit!!
The cell is connected to +5V supply of Atmega16 via 47K.
The other end of cell is ground.
Measuring voltage across cell did not give meaningful results.
I tried changing 47K to 200K on higher side in steps, and lower side to 10K.
In all the cases there is very marginal change in readings.
Is the input impedance of the ADC responsible for this as the resistance of the cell with solution is expected to be in the range of 20K to 300K.
Any clue?
Is the electrolyte nature of solution the culprit!!