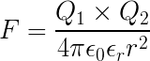Learner
Full Member level 2
- Joined
- Nov 1, 2004
- Messages
- 133
- Helped
- 4
- Reputation
- 8
- Reaction score
- 2
- Trophy points
- 1,298
- Location
- Area 621.xxx
- Activity points
- 1,402
Quote~"If two one-second collections of 1 Coulomb each were concentrated at points one meter apart, the force between them could be calculated from Coulomb's Law. For this particular case, that calculation becomes

The density of metallic copper is about 9 grams/cm3 and one mole of copper is 63.5 grams so the cubic centimeter of copper contains about 1/7th of a mole or about 8.5 x 1022 copper atoms. With one mobile electron per atom, and with the electron charge of 1.6 x 10-19 Coulombs, this means there are about 13,600 Coulombs of potentially mobile charge in one cm3 of copper."
http://hyperphysics.phy-astr.gsu.edu/hbase/electric/elefor.html#c1
I am trying to carry out a simply experiment to witness the electric force however it has been unsucessful, based on my understanding if I connect 2 pieces of metal to the positve terminal of the power supply negative charges on the metal pieces would be drawn into the positive terminal of the PSU. Therefore, becomes postively charged and would repel each other. However, this is not the case which is what I expected. Similarly, if I place the same terminal of the battery towards each other they should repel due to electric force but they don't.
Why? Can anyone help clear up my confusion on this simple problem?
Also, based on electric force concept a battery should exhibit a electric dipole field?


The density of metallic copper is about 9 grams/cm3 and one mole of copper is 63.5 grams so the cubic centimeter of copper contains about 1/7th of a mole or about 8.5 x 1022 copper atoms. With one mobile electron per atom, and with the electron charge of 1.6 x 10-19 Coulombs, this means there are about 13,600 Coulombs of potentially mobile charge in one cm3 of copper."
http://hyperphysics.phy-astr.gsu.edu/hbase/electric/elefor.html#c1
I am trying to carry out a simply experiment to witness the electric force however it has been unsucessful, based on my understanding if I connect 2 pieces of metal to the positve terminal of the power supply negative charges on the metal pieces would be drawn into the positive terminal of the PSU. Therefore, becomes postively charged and would repel each other. However, this is not the case which is what I expected. Similarly, if I place the same terminal of the battery towards each other they should repel due to electric force but they don't.
Why? Can anyone help clear up my confusion on this simple problem?
Also, based on electric force concept a battery should exhibit a electric dipole field?