samic45mit1
Member level 3
Hello,
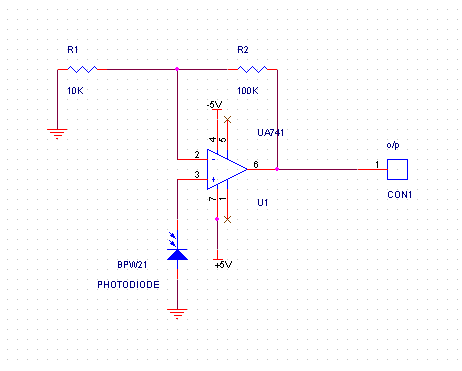
Please help to find sensor for calorimeter. I am using BPW21 ,But it not give result. My wavelength range is 400 to 670 nm .
Thank you
Please help to find sensor for calorimeter. I am using BPW21 ,But it not give result. My wavelength range is 400 to 670 nm .
Thank you